

Fighting a New Flu
A Medical Center scientist helps lead the fight against potential pandemic.
By Scott Hauser | Photography by Richard Baker
 |
| FRONT LINES: The study, led by Medical Center scientist John Treanor (top left), is investigating whether an experimental vaccine can ward off bird flu, which has raised alarms in southeast Asia (bottom left), where infected poultry have been culled to stem the virus’s spread. |
As he settles into his office at the Medical Center each morning, John Treanor ’79M (MD) visits the Google News Web site. There, he enters a term that has occupied his mind for much of the past few years: “bird flu.”
For one of the nation’s leading experts when it comes to combating the viruses behind influenza, the ritual may seem a little unscientific. But the kind of news that the Rochester professor of medicine is looking for may take weeks to show up in the peer-reviewed studies or national health agency reports that he typically reads.
He, like many in the medical community around the world, is keeping close tabs on a foe that can move quickly, hopping across borders in a matter of days, if not hours. So every little bit of information helps, especially daily news reports from Vietnam, Thailand, Hong Kong, and other places in southeast Asia where, as of last spring, at least 50 people had died from bird flu, also known as “avian flu.”
While those numbers may seem small, they represent what many in the international system designed to track and respond to global health threats see as the tiny tip of a looming infectious disease iceberg. The most troublesome virus—identified as H5N1, according to the classification system used to name flu strains—has prompted normally hedge-betting scientists to talk in terms of a coming global pandemic, with some making comparisons to the kind of scourge that has not been seen since 1918.
“The likelihood of a flu pandemic in the next five years is significantly greater than the likelihood of a terrorist attack using an infectious agent like anthrax or smallpox,” says Treanor. “These things are out there, and we need to take them seriously. You can’t panic, but you have to be prepared as well.”
Treanor, director of the Vaccine Treatment and Evaluation Unit at the Medical Center, is leading a key component of that preparation. As one of seven sites across the country funded through the National Institutes of Health to conduct clinical studies of vaccines, the center has for more than two decades been on the front lines in the nation’s fight against infectious diseases like the flu (as well as, in recent years, the potential bioterrorism threats of anthrax and smallpox).
In April, Treanor’s lab began testing a potential vaccine against bird flu, making the Medical Center the lead institution for a three-site, 450-person study sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). Preliminary results released in August indicated that the vaccine is effective at stimulating immunity against the virus, although further tests will be conducted to fine-tune those findings.
“These things are out there, and we need to take them
seriously. You can’t panic, but you have to be prepared as well.”
—Treanor
That’s about one third of the time allotted for most flu vaccine trials—“That’s about as fast as you can do something like this,” Treanor says—and it indicates some of the urgency driving the concern over bird flu.
“The virus has the potential to trigger the next pandemic, which, judging from history, is well overdue,” noted Anthony Fauci, director of the NIAID, in the journal Nature last May. “We cannot predict exactly when a pandemic will occur, nor can we know for certain whether the culprit will be H5N1 or a related virus. The only certainty is that it will present extraordinary challenges.”
“Clearly, there is much to be accomplished, and time is of the essence.”
If the urgency troubles him, Treanor, a licensed pilot who recently took up classical guitar, doesn’t let on as he strolls through the unit on the fourth floor of Strong Memorial Hospital. In a hallway not far from Golisano Children’s Hospital, the unit’s staff of seven full-time nurses guide study volunteers from exam rooms to waiting areas and back again with a practiced efficiency.
At any one time, the unit may have as many as five clinical trials of vaccines against infectious agents in various stages of completion. As the team inoculates participants in the bird flu vaccine study, they are recruiting volunteers for a study testing a vaccine against herpes in women.
“We’re very good at getting things done fast with a lot of data and a lot of accuracy,” Treanor says matter-of-factly.
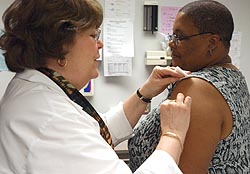 |
| Nationwide, about 450 people like Ardel Taylor of Rochester (right) volunteered to take part. |
Efficiency is a virtue in the effort to stay ahead of the annual assault of influenza, a disease that each year kills an estimated 36,000 Americans and sends another 200,000 to the hospital. While those at the edges of the life spectrum—the elderly and infants—are most at risk, the disease’s body slam of high fever, cough, and joint pain can lay low even the most physically fit.
In the northern hemisphere, the brunt of the season usually hits in November and lasts through March. In order to be prepared with a vaccine, scientists rely on data from southeast Asia where, because of the seasonal differences between the two hemispheres, the flu season arrives six months earlier than it does north of the equator.
The information has to be ready by January so that there’s enough time to evaluate the strains, identify the most worrying ones, and manufacture a vaccine, a process that can take six to eight months. The closer the vaccine matches the expected strain, the better it will be at triggering antibodies in the human immune system. That, in turns, helps the body ward off the disease or at least lessen the infection’s impact.
Although that schedule leaves little room for error—or, as occurred in 2004, a sudden loss of a vaccine supplier—the system generally runs pretty smoothly, according to Treanor. In most seasons, the focus is on pinpointing slight variations among strains that have been infecting humans for the past 30 years.
That’s why the virus behind avian flu troubles scientists.
Volunteers Needed
With as many as five studies under way at any one time, the Vaccine Treatment
and Evaluation Unit at the Medical Center has one ongoing need: more volunteers.
As the 150 participants in Rochester (out of 450 nationwide) were enrolled in
the study for a bird flu vaccine, the unit also was recruiting other volunteers
for a vaccine against herpes in women. Then there were the studies for vaccines
against smallpox and anthrax that were winding down.
“We’re constantly looking for more volunteers,” says Diane
O’Brien, the clinical coordinator of the unit.
Volunteers generally need to be healthy adults between the ages of 18 and 64,
but some studies have more specific parameters. Most pay a small fee to offset
the costs of having to visit the Medical Center several times over the course
of a study.
For more information, contact the unit at (585) 273-3990.
—Scott Hauser
“Bird flu is an extreme example of a new strain of flu,” Treanor says. “No one has ever had it before. Unlike a typical flu virus, which most people have some immunity against, people have no defense against bird flu.”
Unchecked, the result would be the worldwide spread of the disease. Since the first was documented in 1890, flu pandemics have occurred about every 30 or 40 years, with the most notorious in 1918 when between 20 million and 100 million people are estimated to have died around the world.
Each pandemic has been attributed to a major change in the molecular makeup of the flu virus, what virologists call an antigenic shift.
“The flu is very much a moving target,” Treanor says.
Ever evolving, flu viruses are organisms smaller than a single cell and contain eight genetic pieces that provide the instructions for replicating themselves. That information is encased in two protein surfaces: one, called hemagglutinin (HA), makes the virus stick to other cells, and the other, called neuraminidase (NA), allows newly replicated viruses to escape to attack other cells.
Each protein has evolved into subtypes—there are 15 different HA subtypes and 9 NA subtypes—that can combine to form an array of flu strains roughly identified by their HA and NA subtypes. For example, the 1918 pandemic was caused by the H1N1 virus, while the much less devastating 1968 pandemic was caused by the H3N2 virus.
Since then, nearly each seasonal flu virus has been a member of either the H1 or H3 strain, and the process for developing vaccines against them has been fairly routine.
The virus behind bird flu, which was first identified in chickens in Hong Kong in 1997, is something new. Until a handful of people who worked with poultry became infected in 1997 and 1998, the H5 strain had never been seen in humans.
In some pockets where humans have been infected, the mortality rate has reached 70 percent. For the “typical” flu, the mortality rate is less than 1 percent.
And although as of this summer, the new virus had yet to demonstrate that it could easily pass from one human to another, Treanor notes that each of the major pandemics of the last century—including 1918—were caused by mutations in the HA subtype. And each change was first identified in poultry, which are common carriers of flu virus.
Scientists fear that someone with a “normal” flu strain could become infected with the H5 subtype, giving the virus a metaphorical laboratory to mutate again into a form that could easily be transmitted from one person to another.
“The resulting disease could be very severe, and it could spread very rapidly,” Treanor says.
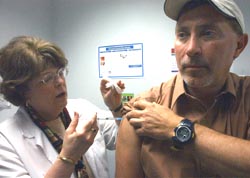 |
| CLINICAL TRIAL: Nurse Barbara Fernaays administers the vaccine to volunteer Edward Dunlop of West Seneca, New York. |
Although he’s confident that a vaccine can be developed to fight H5N1 effectively, the ability of the United States and other countries to respond quickly with enough vaccine is extremely limited.
The vaccine being used in the study was developed after researchers at St. Jude’s Children’s Hospital in Memphis “reverse engineered” the virus’s molecular structure. As part of the Rochester study, Treanor’s team is trying to determine an effective dosage level that will trigger enough antibody response to fend off the virus.
Depending on what that study shows, the National Institutes of Health have a supply of vaccine for between 2 million and 8 million doses. That’s enough to begin vaccinating health care workers, but few others would get the vaccine until more could be manufactured. The United States would require on the order of 750 million doses, enough for each person to receive the two inoculations necessary for the vaccine to take full effect.
If the virus figures out the human-to-human transmission barrier, there would be little time to waste, Treanor says.
What about the ‘Regular’ Flu?
Get your flu shot. That’s the advice of John Treanor, who in addition
to directing the Medical Center’s Vaccine Evaluation and Treatment Unit
is a physician in the Department of Internal Medicine at Strong Memorial Hospital,
where he treats patients with infectious diseases like the flu.
The 2005–06 season is expected to be a “normal” year when
it comes around again in November, and the vaccine for the expected strain—a
variation of the H3 subtype—should be ready, he says.
He realizes that some people may be skeptical about getting a shot because of
last year’s supply disruption when a plant in Great Britain owned by the
pharmaceutical company Chiron was forced to suspend production, but he says
the evidence is overwhelming that vaccines greatly reduce each season’s
severity.
According the National Institute of Allergy and Infectious Diseases, vaccines
are effective at thwarting infection in about 60 percent of people older than
65 and about 70 percent of people below that age.
—Scott Hauser
“That’s the Achilles heel of pandemic planning: In almost any scenario you can come up with, it’s too late to prevent the first wave of cases,” Treanor says.
Another option would be to go ahead and manufacture enough vaccine to inoculate people against bird flu. That might be some help, Treanor says, but it would be limited because H5N1 will most likely mutate slightly as it becomes adept at infecting humans, limiting the immunity provided by the first vaccine.
“It’s not possible to vaccinate ahead of time against virus strains that haven’t yet occurred,” he says.
Nor does he put much stock in calls by some policymakers for countries to stockpile the antiviral drug sold under the name Tamiflu. Developed in the past half-decade, the drug has shown some efficacy in lessening the severity of flu symptoms, but it must be administered shortly after infection. As of this spring, the nations most at risk for infection from bird flu had few doses of Tamiflu on hand.
A vaccine, a defense that has proven itself over the past half century, is the best hope, he says.
In a small stretch of hallway, just off one of the main hallways of Golisano Children’s Hospital, where kids wheel IV caddies less than 200 feet away, Treanor’s clinical staff tends to a steady stream of people who have volunteered to come to the hospital in the hopes of helping him in that effort.
People like Jaimie Madsen, a 28-year-old office manager from Rochester, who has participated in several studies for the unit. While she gets a flu shot each fall, she’s never given much thought to the toll that the flu can take.
“If nobody volunteered, we’d never have a vaccine that would keep people from getting sick,” she says.
Ardel Taylor, another volunteer from Rochester, agrees. “If it’s going to help someone, why not give it a try?”
As part of the trial, participants, who must keep track of their daily health on an 8-by-11 inch card called a “memory aid,” return regularly to have their health checked and to receive second doses. They don’t know how much vaccine, if any, they receive during the trial.
For his part, Treanor puts the odds of a pandemic at between 1 percent and 10 percent within five years, but he says that the odds will change depending on how rapidly H5N1 demonstrates it can mutate and how quickly a vaccine can be readied.
“In my heart of hearts, I don’t expect there to be a pandemic of H5, but we certainly need to be prepared for the possibility,” he says.
“You shouldn’t be staying up at night worrying about it, but it’s something to pay attention to.”
Scott Hauser is editor of Rochester Review.
