

Biomedical Engineering
Building a Better Molecular ‘Mousetrap’
Rochester scientists create a new device to capture and manipulate stem and cancer cells using the hydrodynamics of moving blood.
By Jonathan Sherwood ’04 (MA)
Mike King ’95 is building a better mousetrap. Inside a mouse. Inside an artery.
The trap—a simple tube lined with special proteins—is an implantable device that can pluck selected cells right out of the bloodstream. And King, an associate professor of biomedical engineering, has demonstrated that the new instrument can pull adult stem cells from a rat with a far greater purity than any present technique.
“It’s the kind of research that, before we tried it, we never would have expected such a remarkable result straight out of the gate,” says King.
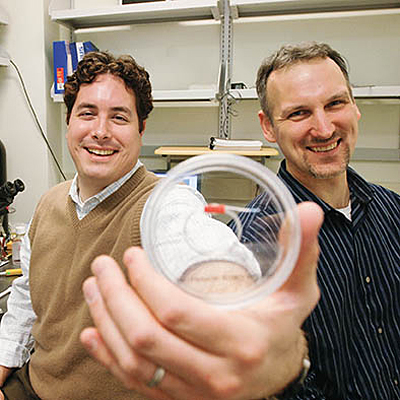
CELLULAR FOCUS: Mike King ’95 (left), an associate professor of biomedical engineering, and Joel Wojciechowski ’92, ’05 (PhD), a postdoctoral fellow, have developed a way to manipulate stem cells and cancer cells by exploiting the mechanics of the cells’ movement.
He is at the forefront of a new field: manipulating stem cells, white blood cells, and cancer cells by exploiting the mechanics of the cells’ movement with such precision that he’s having success capturing and reprogramming several cell types as they pass through the device.
“We’re playing off the hydrodynamics of moving blood to isolate and manipulate specific cell populations with great efficiency.”
In 2002, King was studying how certain white blood cells, called neutrophils, know how to migrate to a point of infection. He observed how, near an injury site, the walls of the nearby blood vessels expressed an adhesive protein called a selectin, and if passing neutrophils brushed against the selectins, they stuck to the vessel wall.
But the cells did not remain stuck—they rolled. With a precise balance between the adhesion and the forces of the flowing bloodstream, the cells could move much more slowly as they approached the infection site.
With that slowed pace, the cell can look for a second signal on the vessel wall that tells the cell to exit the vessel by squeezing between cells in the wall and moving directly to the site of infection.
One reason the system is so effective is that only neutrophils respond to the selectins, so only neutrophils slow down in the blood.
King was working out the physical dynamics of this neutrophil rolling in his office one day when Jane Liesveld, a hematology clinician doing work on bone marrow stem cells at the Medical Center, walked by and noticed a poster of King’s work in the hallway.
“She dropped in and said, ‘I have a pretty plentiful source of primary stem cells from patients. Can you think of any biophysical research to do with that?’” says King. “The stem cell angle just fell from the sky.”
As King worked with Liesveld he found that the basic rolling mechanism was the foundation of a number of other processes, including stem cell transplantation—a natural phenomenon where stem cells move in and out of bone tissue via the blood. In 2004, he found that he could coat a material with specific adhesive selectins and capture living stem cells.
Now King and colleagues have shown, in a March paper in the British Journal of Haematology, that they can take the process a step further. When they implanted the device in a rat, the selectin coating remained active for one to two hours.
When the tube was removed, King found he’d indeed captured cells straight out of the bloodstream, including contaminants—non-stem cells—as expected. What he didn’t expect was how many of the cells were viable stem cells.
“I was astounded,” says King. “More than 25 percent of the sample was stem cells. It’s amazing because even when you use drugs to increase the number of free stem cells in the blood, they still only make up less than 1 percent of all cells. If you use traditional methods to collect stem cells, centrifuging the rat’s blood, even in these drug-treated rats you might get 3 or 4 percent stem cells—meaning only 3 or 4 percent of the cells you obtain are stem cells.”
King points out that centrifugal methods currently produce an overall higher stem cell yield because they start with far more blood, but he believes his microscale device can operate at a significantly larger capacity.
King is even more enthusiastic about his work in reprogramming cells that pass through his device. As a cell rolls across the adhesive surface, it can be forced to contact other proteins. King says the proteins can be designed to steer a stem cell’s development, forcing it to become a specific type of blood, bone, or muscle cell.
King hopes someday an implantable device could continuously reprogram errant neutrophils, but he is already at work on a device that holds the same promise for cancerous cells.
Cancer cells use the same rolling mechanism to travel around the body and lodge in interstitial tissue, so King is focused on isolating the selectins that cancer cells respond to. His lab is working to create a microscale tube that might attract cancer cells and use receptor-mediated triggering proteins to reprogram the cells to self-destruct.
With his microscale tube device, King has already verified that he can control the rolling adhesion of various types of cancer cells, including leukemias, prostate, retinoblastoma, and colorectal cancer cells.
“One of our ultimate goals is to develop an implantable device that will selectively remove metastatic cells from the blood,” says King. “Those cells can predate detectable tumors by years, so we might catch them before they become dangerous.”
