Public Health
Retrospective Analysis of the relationship between Obstructive Sleep Apnea and Nocturnal bradycardia
Fall 2024, Volume 23, Issue 1
Sophia Nguyen ’27, Jagdish Patel*
https://doi.org/10.47761/ZTSU1276
OSA can be classified as mild, moderate, or severe based on the apnea-hypopnea index (AHI) system. The AHI is calculated as the average number of apneas and hypopneas per hour of sleep, and is one of several metrics reported during a polysomnography test (sleep study) in addition to brain activity, heart rate, oxygen saturation, and muscle movements (Somers et al., 2008). The American Academy of Sleep Apnea delineates mild apnea as 5-15 events per hour, moderate apnea as 15-30 events per hour, and severe apnea as 30 or more events per hour (reference Figure 2). Treatment of OSA varies from lifestyle modifications with an emphasis on weight loss, to oral appliances such as continuous positive airway pressure (CPAP) machines, surgeries such as removal of tonsils and adenoids through Uvulopalatoplasty, and hypoglossal nerve stimulation (Pavwoski & Shelgikar, 2017).
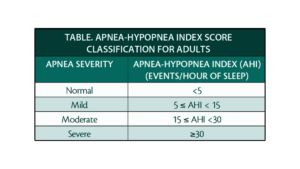 Figure 2: The AHI scoring system
Figure 2: The AHI scoring system
The AHI has been the standard metric to quantify OSA severity for several decades. However, recent studies suggest that the AHI severity grading system may have inherent pitfalls (Malhotra et al., 2021). The system classifies apnea and hypopnea as equal events, despite significant differences in their physiological effects. Variation in definitions of hypopnea may also give rise to inconsistent measurements, and therefore cause discrepant calculations of final AHI scores. (Robards, n.d.). The AHI system also fails to account for body position duration, how long someone remains in a position like sitting or laying down, during apneic events, which may reduce overall scoring accuracy (Soori et al., 2022). The aforementioned findings collectively express that AHI as a standalone for severity classification may lack nuance and oversimplify the categorization of OSA. Consequently, OSA may be misclassified, causing key variables influencing patient health status to be overlooked which impacts clinical outcomes as well as treatment strategy choices
As emerging evidence suggests limitations to the AHI system, more attention is being focused toward broader clinical complications and conditions comorbid with OSA. Physical obstruction of the upper airway hinders the flow of oxygen during sleep, resulting in OSA becoming an associated underlying co-morbidity in numerous non-communicable, chronic conditions such as cardiovascular disease (CVD), hypertension, stroke, cardiac arrhythmias, and mental health-related disorders such as depression and anxiety (DiCaro et al., 2024). The association between OSA and cardiovascular disease is widely known, with growing evidence suggesting that OSA may exacerbate cardiovascular conditions through a variety of physiological mechanisms (Tietjens et al., 2019). The incidence and prevalence of OSA are of epidemic proportions among patients at risk for Sudden Cardiac Death or CVD, which Blackwell et al. in 2019 attributed to several shared risk factors (reference figure 1).
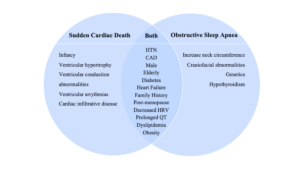 Figure 1: Individual and shared risk factors of sudden cardiac death and obstructive sleep apnea
Figure 1: Individual and shared risk factors of sudden cardiac death and obstructive sleep apnea
Despite its high comorbidity with cardiovascular disease, OSA remains both underdiagnosed and undertreated (Yeghiazarians, 2021). As studies continue to connect CVD and OSA while others reject the AHI system as the sole standard for OSA categorization, it is essential to develop classification mechanisms that are not only accurate, but also multidimensional. The limitations of the AHI system may contribute to the underdiagnosis and undertreatment of OSA within patients who have CVD. For instance, Suen et al. in 2020 found that there are “significant knowledge gaps regarding the effect of treatment and OSA severity” on cardiovascular outcomes due to existing scientific literature only comparing the prevalence of CVD in OSA patients and vice versa.
Given that OSA is a multifaceted condition, this study aims to identify another quantifiable surrogate that serves as an adjunct for the current AHI system. Existing literature across diverse medical fields within internal, cardiovascular, and sleep medicine commonly acknowledge that there is a high prevalence of OSA within patients who have nocturnal bradycardia. A notable meta-analysis in 2022 quantified the occurrence of OSA in patients with nocturnal bradycardia, and vice versa, as well as the effectiveness of the CPAP (Continuous Positive Airway Pressure) machine in addressing the bradyarrhythmia. Cumulative incidence rates and prevalence were tracked through several statistical analysis platforms. Among the 34 articles used, 4852 patients were identified and used for the analysis. The prevalence of both daytime and nocturnal bradycardia was approximately 25% and 69.8% respectively. Within the patients who had bradyarrhythmia, the prevalence of OSA was approximately 56.8%. CPAP treatment did not seem to significantly impact the prevalence/occurrence of daytime or nocturnal bradycardia (Teo et al., 2022). Researchers have connected the two conditions together and conducted studies to display how sleep apnea gives rise to bradycardia in the night. Zwillich et al. (1982) investigated the potential mechanisms eliciting bradycardia during sleep apnea, and how vagal efferent activity was a common trigger for bradycardia amongst enrolled subjects. This seminal study was the first to evaluate heart rate variability amongst patients with sleep apnea. Along with heightened vagal activity, it has been discovered that sleep apnea gives rise to nocturnal bradycardia, through the snowball effect beginning with the blocking of lung expansion. Rossi et al. in 2013 evaluated the “Effects of Sleep Apnea and Heart Rhythm”, further affirming that the upper airway prevents lung expansion and stretching of vagolytic fibers in the lung.This elicits the diving reflex, which then increases sympathetic vasoconstriction to muscles and viscera to maintain perfusion to the vital organs. The result of the cascade is a rise in blood pressure and vagally-induced reflex bradycardia. With clear scientific connections to OSA – as well as a high prevalence within OSA patients – nocturnal bradycardia may be an appropriate and useful cardiovascular condition to potentially contribute to earlier and more accurate OSA diagnosis and classification.
Patients diagnosed with daytime or nocturnal bradycardia may receive a loop recorder, a device inserted under the skin that monitors heart rhythm and rate continuously. Cardiac pacemakers may also be implanted to pace the heart if the rhythm reaches a rate that is below the set threshold rate. As of yet, current research does not establish a correlation between pacemaker therapy and alleviation of OSA symptoms.
It was hypothesized that the degree of nocturnal bradycardia coupled with the AHI system increases the specificity of severity grading for OSA. It was also expected that patients who benefit clinically and physiologically have less frequency and duration of nocturnal bradycardia with optimal therapy for OSA.
RESEARCH DESIGN AND METHOD
Study Design
The purpose of this study was to assess nocturnal bradycardia as a potential indicator for OSA severity grading within cardiac patients. A retrospective pilot observational study was conducted to assess the association between nocturnal bradycardia and OSA in cardiac patients who meet established criteria for OSA treatment and event monitoring (Medicaid Coverage Database, n.d.).
Data Acquisition
Pre-existing data was extracted and analyzed from AthenaHealth electronic medical records (EMRs) to minimize risk to subjects and to efficiently assess any trends pertaining to nocturnal bradycardia events and OSA pathogenesis. Data abstraction took place after obtaining informed consent from the cardiology clinic and respective patients enrolled. Printed informed consent forms were provided to all enrolled patients. Each form provided a description of the study, the data being acquired, and how the data will be used to establish conclusions or relationships between OSA and nocturnal bradycardia. All patient data abstraction processes de-identified personal health information to maintain patient confidentiality.
Through chart abstraction, relevant data (socio-demographic, clinical characteristics, patient characteristics, event monitor and sleep study results) were analyzed and extracted for the purpose of this study (reference figure 3). Race, gender, age, comorbidities, current medications, functional status (New York Heart Association Classification), alcohol and tobacco use, and family history were all reported as patient factors. AHI scores, and for some subjects, oxygen saturations, and heart rate were extrapolated from sleep study data. Additional metrics for certain patients were reported on the sleep study such as time spent under 90% Oxygen saturation, and were therefore listed in this study. Nocturnal bradycardia was defined according to the American Heart Association position statement as a heart rate of less than 60 beats per minute (Kusumoto et al., 2018). To determine the prevalence of Nocturnal bradycardia, event monitor data in the form of device interrogations were utilized to display continuous monitoring of the patient’s rhythm during a specified time period.
The process of care for patients with event monitors and OSA was quantified by concurrent formal guidelines based on the American College of Cardiology (Kusumoto et al., 2018) and American Association of Sleep Medicine (Kapur, 2017) for the diagnosis of Nocturnal bradycardia and obstructive sleep apnea.
Participant selection
This study enrolled all consecutive patients who have a formal diagnosis of OSA (n=5), and fulfill other inclusion criteria such as an event monitor implanted from an outpatient cardiology office setting. Given that this study is a pilot study, the association between OSA and nocturnal bradycardia is still being explored experimentally and the inclusion criteria of both conditions are highly specific. Five cases were chosen to be analyzed. Although sample size was limited, the purpose of this study was to establish and assess baseline trends among patients with both OSA and nocturnal bradycardia, which could be tested in larger cohort studies.
The inclusion criteria for this study is as follows:
- The patient is diagnosed with OSA and currently being monitored with a 30-day event monitor (AHI > 5.0)
- The patient is willing and able to give informed consent for their clinical and background data to be examined
Exclusion criteria included non-English speaking patients to maximize efficient communication as well as patients who could not give informed consent to participating in this study. No expectation for early withdrawal was anticipated, given the observational nature of this study. However, a patient was able to withdraw from the study at any time.
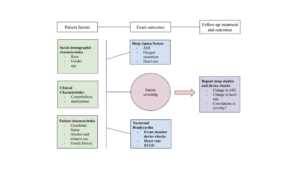 Figure 3: Flowchart illustrating the relationship between various subject factors and exam outcomes in sleep apnea assessment. Subsequent evaluations, such as repeat sleep studies and device interrogations, were used to monitor changes in apnea severity, heart rate, and oxygen saturation to evaluate potential relationships between nocturnal bradycardia and OSA.
Figure 3: Flowchart illustrating the relationship between various subject factors and exam outcomes in sleep apnea assessment. Subsequent evaluations, such as repeat sleep studies and device interrogations, were used to monitor changes in apnea severity, heart rate, and oxygen saturation to evaluate potential relationships between nocturnal bradycardia and OSA.
RESULTS
Of the five selected subjects for the study, three underwent facility-structured sleep studies, and the remaining two subjects participated in an in-home sleep study. All patients were diagnosed with OSA based on having a minimum AHI of 5.0. Each subject also had an implantable loop recorder, which permitted the analysis of device checks including bradycardia events. A de-identified description of each subject’s clinical profile is presented, containing information such as major cardiac events, medications, family history, and compliance. Loop recorder implantation dates and sleep study dates were recorded to serve as baseline patient data. Device checks on loop recorders are displayed over time to convey potential relationships between OSA and Nocturnal bradycardia. Milestone tables and supporting visuals are also included to convey possible trends.
Subject A
Subject A is a 72-year-old Caucasian male with comorbidities including paroxysmal atrial fibrillation, bilateral carotid disease status post left carotid endarterectomy, essential hypertension, severe aortic valve stenosis, status post transcatheter aortic valve replacement, and embolic stroke. The subject had an in-home sleep study on May 19, 2015 and was subsequently diagnosed with OSA (AHI of 6.5). The patient refused OSA therapy, but received a loop recorder implantation on January 28, 2022, which detected several bradycardic episodes.
An asystolic episode of 4.4 seconds occurred on February 4, 2023, at 5:03 AM during sleep. The loop recorder started to detect atrial fibrillation in July of 2022. The patient had a repeat in-home study in early March, which demonstrated an AHI of 18 (nearly a three-fold increase in AHI since diagnosis). The patient has been prescribed the following medications: Sotalol (80 mg twice daily), Losartan (100 mg daily), Atorvastatin (80 mg at night), Amlodipine (10 mg daily), Pantoprazole (40 mg daily), and Eliquis ( 5mg twice daily). The patient’s mother has a pacemaker and a history of hypertension and atrial fibrillation, and his father had a coronary artery bypass graft to treat coronary artery disease. His functional status has no limitations, and he does not smoke or drink alcohol.
| Date | Event |
| 01/04/2022 | Sinus rhythm (63 bpm) without any abnormalities |
| 02/28/22 | Sinus rhythm (no nocturnal bradycardia or atrial fibrillation recorded) |
| 07/09/2022 (device check) | 4 days of intermittent atrial fibrillation with a mean heart rate of 78 bpm (no nocturnal bradycardia recorded) |
| 07/20/2022 | Atrial fibrillation detected |
| 08/05/2022 (device check) | Atrial fibrillation recorded at 5 AM with a heart rate of 26 bpm |
| 01/25/2023 | 6 nocturnal bradycardia events recorded with 140 pauses. An atrial fibrillation episode of 1 hour and 3 minutes results in an atrial fibrillation burden of 2% |
| 02/04/2023 | At 5:03 PM (while the subject naps) there is a 4.4 second long pause |
| 02/05/2023 – 03/07/2023 | Over 40 asystolic events are detected |
| Early March (no specific date provided) | An AHI of 18 is recorded |
Table 1 – Subject A’s Milestone table
| AHI | 65 |
| ODI (1/hr) | 3.9 |
| Mean Oxygen Saturation | 95% |
| Min Oxygen Saturation | 79% |
| Mean Heart Rate (bpm) | 59 |
| Minimum Heart Rate | 54 |
| Maximum Heart Rate | 115 |
| Time spent under 90% Oxygen Saturation | 2.6% |
Table 2 – Subject A’s initial sleep study data
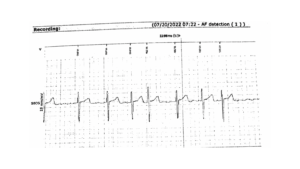 Figure 4 – An EKG on 07/20/2022 displays a baseline rhythm of atrial fibrillation
Figure 4 – An EKG on 07/20/2022 displays a baseline rhythm of atrial fibrillation
 Figure 5 – The patient has a 4.4 second pause on 02/04/2023 at 5:03 PM (during a nap)
Figure 5 – The patient has a 4.4 second pause on 02/04/2023 at 5:03 PM (during a nap)
 Figure 6 – A list of 40+ asystolic events detected from 02/05/2023 to 03/07/2023
Figure 6 – A list of 40+ asystolic events detected from 02/05/2023 to 03/07/2023
Subject B
Subject B is a 63-year-old Caucasian male with comorbidities including coronary artery disease, coronary stent placement involving the left anterior descending (LAD) artery, permanent atrial fibrillation, essential hypertension, obesity (BMI of 37), hyperlipidemia, and congestive heart failure (Diastolic) Class II. The patient had a facility-structured polysomnography test on August 28th, 2011. He was subsequently diagnosed with OSA (AHI of 14). The patient was prescribed a CPAP machine and was initially noncompliant with therapy. The patient received a loop recorder implantation on December 7th, 2021. After March 10th, 2022, the patient became compliant with CPAP therapy and medicines. The patient has been prescribed the following medications: Metoprolol Tartrate (75 mg twice daily), Losartan ( 25 mg daily), Brilinta (90 mg twice daily), Xarelto (20 mg nightly), Atorvastatin (20 mg nightly), and Glimepiride (2 mg twice daily). The patient’s grandmother has a heart disease history and a pacemaker. His functional status has no limitation, and he does not smoke or drink alcohol.
| Date | Event |
| 12/07/2021 | The subject’s baseline rhythm of chronic atrial fibrillation is recorded |
| 12/20/2021 | Permanent atrial fibrillation is detected with a ventricular response of 80 bpm |
| 02/27/2022 | At 5:43, the subject is napping and experiences a sudden rate drop with a 4.5 and 6.4 second pause |
| 03/10/2022 (device check) | A heart rate of 27 bpm is recorded |
| 03/03/2023 | At 8:30 PM a heart rate of 60 bpm is detected. The rate decreases from 60 bpm to 48 bpm without any asystolic events |
| 03/09/2023 | No pauses are detected |
Table 3 – Subject B’s Milestone table
| AHI | 14 |
| Min Oxygen Saturation | 88% |
Table 4– Subject B’s initial sleep study data
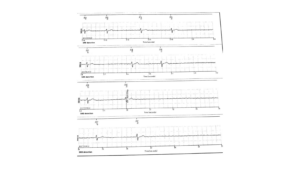 Figure 7 – Lowest heart rates for Subject B
Figure 7 – Lowest heart rates for Subject B
Subject C
Subject C is a 53 year old Caucasian male with comorbidities including diabetes mellitus type I, morbid obesity (BMI of 46), essential hypertension, chronic kidney disease stage III, and chronic diastolic heart failure. The patient had an in-home sleep study on April 6th, 2021. He was subsequently diagnosed with OSA (AHI of 44). After being diagnosed with OSA, the patient was prescribed an oral appliance. Due to jaw pain, the patient abandoned therapy at three months of use and switched to CPAP therapy. The patient was also compliant with medicines but non-compliant with his CPAP. He also received a loop recorder implantation on November 10th, 2021. The patient has been prescribed the following medications: Brilinta (90 mg twice daily), Aspirin (81 mg daily), Fenofibrate (160 mg nightly), Bumetanide (1mg daily), Amlodipine (10mg daily), Hydralazine (100 mg thrice daily), and Oxybutynin Chloride (5mg nightly). The patient’s mother had coronary artery disease and a coronary stent; his father has a history of coronary artery disease with a coronary stent and has had atrial fibrillation ablation. His functional status is Class II – III, and he does not smoke or drink alcohol.
| Date | Event |
| 11/22/2021 | Nocturnal bradycardia is detected at 4 AM with a heart rate of 47 bpm. At 4:27 AM, the patient has a 2.2 second asystolic event. |
| 02/02/2022 | At 4 AM the patient has a 2 second pause and a 5.4 second pause at 2:07 AM. |
| 07/28/2022 | The patient had a heart rate of 20 bpm for 20 seconds at 3:31 pm. At this time, the patient was napping. |
| 01/12/2023 | At 2:53 PM the patient had an asystolic episode of 4.5 seconds. |
| 02/20/2023 | The patient has another sleep study. An AHI of 88.8 is recorded. The subject spent 55.6 minutes below 88% oxygen saturation and had a mean heart rate of 44. The patient was prescribed a CPAP machine. |
| 03/21/2023 | No nocturnal bradycardia or asystole is detected. AHI is recorded to be 5 from a repeat sleep study test. |
Table 5 – Subject C’s Milestone Table
| AHI | 44 |
| ODI (1/hr) | 61.4 |
| Mean Oxygen Saturation | 92% |
| Min Oxygen Saturation | 67% |
| Mean Heart Rate (bpm) | 63 |
| Min Heart Rate | 45 |
| Max Heart Rate | 83 |
| Time spent under 90% Oxygen Saturation | 18% |
Table 6 – Subject C’s initial sleep study data
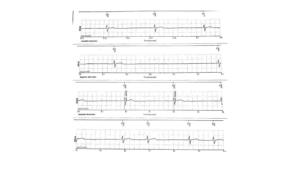 Figure 8 – On 01/12/2023 2:53 PM, Subject C has a 4.5 second pause
Figure 8 – On 01/12/2023 2:53 PM, Subject C has a 4.5 second pause
Subject D
Subject D is a 64-year-old Caucasian male with comorbidities including paroxysmal atrial fibrillation, morbid obesity (BMI of 31), chronic diastolic heart failure Class II, syncope, hyperlipidemia, essential hypertension, and diabetes mellitus type II. The patient had a facility polysomnography test on December 26th, 2016. He was subsequently diagnosed with OSA (AHI of 69) and due to his cleft palate, was prescribed a CPAP machine over an oral appliance. The patient received a loop recorder implantation on December 1st, 2022. The patient has been prescribed the following medications: Xarelto (20 mg nightly), Doxepin (50mg daily), Metoprolol Tartrate (12.5 mg daily), Propafenone (225 mg thrice daily), Fenofibrate (134 mg daily), Lexapro (20mg daily), Metformin (500mg twice daily), and Atorvastatin (20 mg daily). The patient’s mother has a history of terminal lung cancer. His functional status is Class II, and according to his record, quit smoking in 2013.
| Date | Event |
| 12/01/2022 | In office check: At 2:24 PM, the patient has a baseline heart rate of 63 bpm (baseline) |
| 12/02/2022 | At 7 AM, the patient has a heart rhythm of atrial fibrillation with a heart rate of 147 bpm. He suddenly drops to 27 bpm while sleeping. |
| 02/21/2023 | A remote device check detects a long atrial fibrillation episode of approximately two hours, with the maximum heart rate being 152 bpm. |
| 04/06/2023 | At 4:54 AM, atrial fibrillation was detected at approximately 160 bpm for an hour and 54 minutes. |
| 04/08/2023 | At 8:44 PM, atrial fibrillation was detected at approximately 140 bpm. |
Table 7 – Subject D’s Milestone table
Subject E
Subject 5 is a 74 year old caucasian male with comorbidities including ventricular tachycardia, cardiac arrest, COVID-19 infection, overweight (BMI of 28), essential hypertension, paroxysmal atrial fibrillation, and palpitations. The patient had a facility-structured polysomnography test on September 10th, 2019. He was subsequently diagnosed with OSA (AHI of 10.5) and prescribed a CPAP machine that he was only intermittently compliant with. The patient was admitted to a local hospital on July 21st, 2023 and was resuscitated from a cardiac arrest on July 22nd, 2023. The patient had a left coronary angiogram and a drug-eluting stent was deployed within the ramus coronary artery. The subject had a loop implantation on March 2nd, 2023. The patient has been prescribed the following medications: Amiodarone (200 mg daily), Atorvastatin (40 mg nightly), Ticagrelor (90 mg twice daily), Eliquis (5mg twice daily), Metoprolol Tartrate (12.5 twice daily), and Lisinopril (10 mg daily). The patient’s father has a history of congestive heart failure, heart disease, and diabetes. His functional status is Class II, and he has a moderate alcohol intake.
| Date | Event |
| 08/03/2022 | At 11:10 PM, the patient has a heart rate of 82 bpm in sinus rhythm. At 4:18 PM, the heart rate decreases to 54 bpm, and at 1:46 AM, the heart rate again decreases to 36 bpm. |
| 03/16/2023 | A baseline rhythm of sinus bradycardia is detected with a heart rate in the 50s. No asystolic events were recorded. |
| 07/22/2022 | The patient has a cardiac arrest from ventricular tachycardia |
| 03/30/2023 | No bradycardia is detected, with the minimum heart rate reaching 50 bpm. Patient reports feeling well. |
Table 8 – Subject E’s Milestone Chart
 Figure 9 – The subject’s heart rates get progressively slower (50 bpm to 30 bpm)
Figure 9 – The subject’s heart rates get progressively slower (50 bpm to 30 bpm)
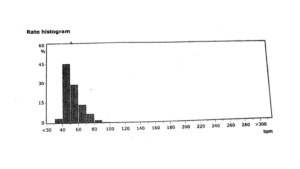 Figure 10 – Subject E’s rate histogram on 03/16/2023 shows bradycardia around 50 bpm and no asystole events
Figure 10 – Subject E’s rate histogram on 03/16/2023 shows bradycardia around 50 bpm and no asystole events
DISCUSSION
The present study is a retrospective observational evaluation designed to elucidate the relationship between the severity of OSA and Nocturnal bradycardia. This study demonstrates the occurrences of bradycardia during periods of apnea amongst the five subjects that were studied. All patients had a formal sleep study with the lowest AHI of 6.5 (Subject A) and lowest oxygen saturation of 79% to the most severe apneic patients with an AHI of 88 (Subject D) with the lowest oxygen saturation of 67%. This study demonstrates several interesting relationships:
- The degree of bradycardia – as recorded with loop technology – appeared to have an association with OSA severity as observed in Subjects A and C. As OSA increased in severity, their heart rates decreased over time.
- There is a potential relationship between severity of bradycardia and OSA treatment compliance as noted in Subjects B and C. Nocturnal bradycardia burden decreased with CPAP adherence (>4 hrs/night). However the degree of CPAP compliance to offset bradycardia was not studied and must be further investigated among larger cohorts to establish clinical significance.
- There is a possible relationship between the presence of atrial fibrillation and OSA severity as observed in Subject A, B, D and E. Unlike other subjects, Subject D had tachycardia (heart rate > 60 bpm) in the setting of atrial fibrillation which is a rhythm that reflects a conduction abnormality. As bradycardia in subjects D and E persisted ( noncompliant with therapy), their atrial fibrillation appeared to worsen.
- Improvement in AHI with reduction in nocturnal bradycardia burden was observed among subjects who had experienced more frequent and prolonged episodes of nocturnal bradycardia burden during the evaluation period, which suggests that the effect of OSA therapy on AHI reduction might be dependent on bodyweight, frequency of and duration of nocturnal bradycardia.
LIMITATIONS
This retrospective study had a variety of limitations:
- Up to date, there are no randomized trials developed to study the potential effects of nocturnal bradycardia on severity of Sleep Apnea based on AHI.
- The sample size was insufficient for robust statistical analysis, and the study demographic lacked females or subjects from other racial groups.
- Medication induced bradycardia may have confounded the data retrieved from Loop interrogation
- Patient’s had different types of sleep studies with only two patients having repeat sleep studies.
- Patients had a variety of OSA therapies which may present additional confounding variables.
- Timing of Loop recorder interrogation was not standardized across all five subjects.
- There were discrepancies between data provided for each patient among sleep studies and device checks due to lack of data available in the EMR.
CONCLUSION
The primary objective of this study was to identify a potential association between the severity of nocturnal bradycardia and AHI. The AHI system is thought to have inherent pitfalls, however, scores may become more accurate and standardized among patients if additional metrics such as nocturnal bradycardia or atrial fibrillation is considered.
Additional randomized studies – with larger sample sizes – using nocturnal bradycardia as a metric in the AHI scoring system may suggest relationships between nocturnal bradycardia and OSA. A randomized study with a larger sample size and demographic that aims to investigate the relationship between nocturnal bradycardia and FDA approved treatments for OSA is another future area for research. In addition, one that highlights the potential effects of the therapy on weight loss, glucose tolerance, heart failure readmission and medical compliance would be beneficial findings.
Supplementing the AHI system with other criteria may lead to earlier and more accurate diagnosis of OSA in the United States, which in turn may lead to earlier intervention and therapy. Earlier treatment can reduce the chances of patients obtaining other cardiovascular comorbidities such as heart disease,therefore reducing the risk of sudden cardiac death. As emerging evidence continues to associate OSA to heart health, it is critical to explore relationships in depth to bridge gaps between dental sleep medicine and cardiovascular practice to potentially reduce mortality.
REFERENCES
- Álvarez, D., Arroyo, C. A., De frutos, J. F., Crespo, A., Cerezo-hernández, A., Gutiérrez-tobal, G. C., Vaquerizo-villar, F., Barroso-garcía, V., Moreno, F., Ruiz, T., Hornero, R., & Del campo, F. (2020). Assessment of nocturnal autonomic cardiac imbalance in positional obstructive sleep apnea. A multiscale nonlinear approach. Entropy, 22(12), 1404. https://doi.org/10.3390/e22121404
- Berry, R. B., Budhiraja, R., Gottlieb, D. J., Gozal, D., Iber, C., Kapur, V. K., Marcus, C. L., Mehra, R., Parthasarathy, S., Quan, S. F., Redline, S., Strohl, K. P., Ward, S. L. D., & Tangredi, M. M. (2012). Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. Journal of Clinical Sleep Medicine, 08(05), 597-619. https://doi.org/10.5664/jcsm.2172
- Blackwell, J. N., Walker, M., Stafford, P., Estrada, S., Adabag, S., & Kwon, Y. (2019). Sleep apnea and sudden cardiac death. Circulation Reports, 1(12), 568-574. https://doi.org/10.1253/circrep.CR-19-0085
- Daccarett, M., Segerson, N. M., Hamdan, A.-L., Hill, B., & Hamdan, M. H. (2008). Relation of daytime bradyarrhythmias with high risk features of sleep apnea. The American Journal of Cardiology, 101(8), 1147-1150. https://doi.org/10.1016/j.amjcard.2007.11.068
- Daoulah, A., Ocheltree, S., Al-faifi, S. M., Ahmed, W., Alsheikh-ali, A. A., Asrar, F., & Lotfi, A. (2015). Sleep apnea and severe bradyarrhythmia – an alternative treatment option: A case report. Journal of Medical Case Reports, 9(1). https://doi.org/10.1186/s13256-015-0596-6
- DiCaro, M. V., Lei, K., Yee, B., & Tak, T. (2024). The effects of obstructive sleep apnea on the cardiovascular system: A comprehensive review. Journal of Clinical Medicine, 13(11), 3223. https://doi.org/10.3390/jcm13113223
- Elwood, P., Hack, M., Pickering, J., Hughes, J., & Gallacher, J. (2006). Sleep disturbance, stroke, and heart disease events: Evidence from the caerphilly cohort. Journal of Epidemiology and Community Health (1979-), 60(1), 69-73.
- Faulx, M. D., Mehra, R., Reis geovanini, G., Ando, S.-I., Arzt, M., Drager, L., Fu, M., Hoyos, C., Hai, J., Hwang, J.-J., Karaoguz, R., Kimoff, J., Lee, P.-L., Mediano, O., Patel, S. R., Peker, Y., Louis pepin, J., Sanchez-de-la-torre, M., Sériès, F., . . . Phillips, C. L. (2022). Obstructive sleep apnea and its management in patients with atrial fibrillation: An international collaboration of sleep apnea cardiovascular trialists (INCOSACT) global survey of practicing cardiologists. IJC Heart & Vasculature, 42, 101085. https://doi.org/10.1016/j.ijcha.2022.101085
- Filchenko, I., Bochkarev, M., Kandinsky, A., Korostovtseva, L., Sviryaev, Y., & Konradi, A. (2020). Continuous positive airway pressure therapy restores bradyarrhythmia with 10-second asystole in hypertensive obese patient with obstructive sleep apnea. HeartRhythm Case Reports, 6(6), 300-303. https://doi.org/10.1016/j.hrcr.2020.02.005
- Garrigue, S., Bordier, P., Barold, S. S., & Clementy, J. (2004). Sleep apnea:. A new indication for cardiac pacing? Pacing and Clinical Electrophysiology, 27(2), 204-211. https://doi.org/10.1111/j.1540-8159.2004.00411.x
- Garrigue, S., Bordier, P., Jaïs, P., Shah, D. C., Hocini, M., Raherison, C., Tunon de lara, M., Haïssaguerre, M., & Clementy, J. (2002). Benefit of atrial pacing in sleep apnea syndrome. New England Journal of Medicine, 346(6), 404-412. https://doi.org/10.1056/NEJMoa011919
- Geovanini, G. R., & Lorenzi-filho, G. (2018). Cardiac rhythm disorders in obstructive sleep apnea. Journal of Thoracic Disease, 10(S34), S4221-S4230. https://doi.org/10.21037/jtd.2018.12.63
- Goweda, R. A., Elsayed, M. E., Rady, A. S., Alnahdi, F. S., Alqurashi, A. M., Alessa, T. T., Bin laswad, B. M., Bin laswad, A. M., Althobity, O. A., Alhothali, A. S., Khafagy, A., Metwally, A., & Zeid, W. (2022). Prevalence of obstructive sleep apnea among patients with cardiovascular diseases. World Family Medicine Journal /Middle East Journal of Family Medicine, 20(9). https://doi.org/10.5742/MEWFM.2022.9525133
- Gunta, S. P., Jakulla, R. S., Ubaid, A., Mohamed, K., Bhat, A., López-candales, A., & Norgard, N. (2022). Obstructive sleep apnea and cardiovascular diseases: Sad realities and untold truths regarding care of patients in 2022. Cardiovascular Therapeutics, 2022, 1-10. https://doi.org/10.1155/2022/6006127
- Holty, J.-E. C., & Guilleminault, C. (2011). REM-related bradyarrhythmia syndrome. Sleep Medicine Reviews, 15(3), 143-151. https://doi.org/10.1016/j.smrv.2010.09.001
- Højager, A., Schoos, M. M., Tingsgaard, P. K., Bock, T. G., & Homøe, P. (2022). Prevalence of silent atrial fibrillation and cardiovascular disease in patients with obstructive sleep apnea. Sleep Medicine. https://doi.org/10.1016/j.sleep.2022.10.002
- Huettner, M., Koehler, U., Nell, C., Kesper, K., Hildebrandt, O., & Grimm, W. (2015). Heart rate response to simulated obstructive apnea while awake predicts bradycardia during spontaneous obstructive sleep apnea. International Journal of Cardiology, 186, 216-218. https://doi.org/10.1016/j.ijcard.2015.03.245
- Jordan, A. S., & White, D. P. (2008). Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respiratory Physiology & Neurobiology, 160(1), 1-7. https://doi.org/10.1016/j.resp.2007.07.009
- Kalantari, E., Kalantari, F., Edalatifard, M., & Rahimi, B. (2022). Evaluating changes in pulse transit time drop index in patients with obstructive sleep apnea before and during CPAP therapy. The Clinical Respiratory Journal, 16(9), 611-617. https://doi.org/10.1111/crj.13532
- Kapur, V. K., Auckley, D. H., Chowdhuri, S., Kuhlmann, D. C., Mehra, R., Ramar, K., & Harrod, C. G. (2017). Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An american academy of sleep medicine clinical practice guideline. Journal of Clinical Sleep Medicine, 13(03), 479-504. https://doi.org/10.5664/jcsm.6506
- Koehler, U., Becker, H. F., Grimm, W., Heitmann, J., Peter, J. H., & Schäfer, H. (2000). Relations among hypoxemia, sleep stage, and bradyarrhythmia during obstructive sleep apnea. American Heart Journal, 139(1), 142-148. https://doi.org/10.1016/S0002-8703(00)90321-1
- Koehler, U., Fus, E., Grimm, W., Pankow, W., Schäfer, H., Stammnitz, A., & Peter, J. H. (1998). Heart block in patients with obstructive sleep apnoea: Pathogenetic factors and effects of treatment. European Respiratory Journal, 11(2), 434-439. https://doi.org/10.1183/09031936.98.11020434
- Krahn, A. D., Yee, R., Erickson, M. K., Markowitz, T., Gula, L. J., Klein, G. J., Skanes, A. C., George, C. F.p., & Ferguson, K. A. (2006). Physiologic pacing in patients with obstructive sleep apnea. Journal of the American College of Cardiology, 47(2), 379-383. https://doi.org/10.1016/j.jacc.2005.09.026
- Kusumoto, F. M., Schoenfeld, M. H., Barrett, C., Edgerton, J. R., Ellenbogen, K. A., Gold, M. R., Goldschlager, N. F., Hamilton, R. M., Joglar, J. A., Kim, R. J., Lee, R., Marine, J. E., McLeod, C. J., Oken, K. R., Patton, K. K., Pellegrini, C. N., Selzman, K. A., Thompson, A., & Varosy, P. D. (2019). 2018 acc/aha/hrs guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Circulation, 140(8). https://doi.org/10.1161/cir.0000000000000628
- Lee, Y.-C., Chang, K.-Y., & Mador, M. J. (2022). Racial disparity in sleep apnea-related mortality in the united states. Sleep Medicine, 90, 204-213. https://doi.org/10.1016/j.sleep.2021.11.014
- Liamsombut, S., Kaw, R., Wang, L., Bena, J., Andrews, N., Collop, N., Stierer, T., Gillinov, M., Tarler, M., Kayyali, H., Katzan, I., & Foldvary-schaefer, N. (2021). Predictive value of sleep apnea screenings in cardiac surgery patients. Sleep Medicine, 84, 20-25. https://doi.org/10.1016/j.sleep.2021.05.007
- Lombardi, C., Faini, A., Mariani, D., Gironi, F., Castiglioni, P., & Parati, G. (2020). Nocturnal arrhythmias and heart‐rate swings in patients with obstructive sleep apnea syndrome treated with beta blockers. Journal of the American Heart Association, 9(21). https://doi.org/10.1161/JAHA.120.015926
- Malhotra, A., Ayappa, I., Ayas, N., Collop, N., Kirsch, D., Mcardle, N., Mehra, R., Pack, A. I., Punjabi, N., White, D. P., & Gottlieb, D. J. (2021). Metrics of sleep apnea severity: Beyond the apnea-hypopnea index. Sleep, 44(7). https://doi.org/10.1093/sleep/zsab030
- Mayo Clinic. (2023, April 6). Sleep apnea. Mayo Clinic. Retrieved September 18, 2024, from https://www.mayoclinic.org/diseases-conditions/sleep-apnea/symptoms-causes/syc-20377631
- Morey, B. N., Ryu, S., Shi, Y., Redline, S., Kawachi, I., & Lee, S. (2022). Associations between sleep apnea risk and cardiovascular disease indicators among chinese and korean americans. Sleep Epidemiology, 2, 100037. https://doi.org/10.1016/j.sleepe.2022.100037
- Oliven, A., Aspandiarov, E., Gankin, I., Gaitini, L., & Tov, N. (2008). Collapsibility of the relaxed pharynx and risk of sleep apnoea. European Respiratory Journal, 32(5), 1309-1315. https://doi.org/10.1183/09031936.00139407
- Pavwoski, P., & Shelgikar, A. V. (2017). Treatment options for obstructive sleep apnea. Neurology Clinical Practice, 7(1), 77-85. https://doi.org/10.1212/cpj.0000000000000320
- Rahimi, B., Tavoosi, A., Sadeghi, T., Arjmand, R., & Edalatifard, M. (2020). Comparison of abnormal heart rate and nocturnal arrhythmia in patients with obstructive sleep apnea and normal subjects. Journal of Sleep Sciences, 4(3).
- Robards, K. (n.d.). AHI and beyond: Exploring alternatives to diagnosing OSA severity. American Academy of Sleep Medicine. Retrieved September 18, 2024, from https://aasm.org/ahi-and-beyond-exploring-alternatives-to-diagnosing-osa-severity/
- Rossi, V., Stradling, J., & Kohler, M. (n.d.). https://pubmed.ncbi.nlm.nih.gov/23258782/. European Respiratory Journal, 1439-1531. https://pubmed.ncbi.nlm.nih.gov/23258782/
- Sasa, Y., Nakai, T., Ikeya, Y., Kogawa, R., Otsuka, N., Kurokawa, S., Nagashima, K., Iida, K., Okumura, Y., & Kunimoto, S. (2022). bradyarrhythmia suspected to be associated with sleep apnea syndrome. International Heart Journal, 63(2), 393-397. https://doi.org/10.1536/ihj.21-517
- Slowik, J., Sankari, A., & Collen, J. (2024). obstructive sleep apnea. In StatPearls. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459252/
- Somers, V. K., White, D. P., Amin, R., Abraham, W. T., Costa, F., Culebras, A., Daniels, S., Floras, J. S., Hunt, C. E., Olson, L. J., Pickering, T. G., Russell, R., Woo, M., & Young, T. (2008). Sleep apnea and cardiovascular disease. Circulation, 118(10), 1080-1111. https://doi.org/10.1161/circulationaha.107.189420
- Soori, R., Baikunje, N., D’sa, I., Bhushan, N., Nagabhushana, B., & Hosmane, G. B. (2022). Pitfalls of AHI system of severity grading in obstructive sleep apnoea. Sleep Science, 15(S 01), 285-288. https://doi.org/10.5935/1984-0063.20220001
- Stegman, S. S., Burroughs, J. M., & Henthorn, R. W. (1996). Asymptomatic brady arrhythmias as a marker for sleep apnea: Appropriate recognition and treatment may reduce the need for pacemaker therapy. Pacing and Clinical Electrophysiology, 19(6), 899-904. https://doi.org/10.1111/j.1540-8159.1996.tb03385.x
- Suen, C., Wong, J., Ryan, C. M., Goh, S., Got, T., Chaudhry, R., Lee, D. S., & Chung, F. (2020). Prevalence of undiagnosed obstructive sleep apnea among patients hospitalized for cardiovascular disease and associated in-hospital outcomes: A scoping review. Journal of Clinical Medicine, 9(4), 989. https://doi.org/10.3390/jcm9040989
- Summer, J. (2023, March 29). Apnea-Hypopnea Index (AHI). Sleep Foundation. Retrieved April 25, 2023, from https://www.sleepfoundation.org/sleep-apnea/ahi
- Teo, Y. H., Han, R., Leong, S., Teo, Y. N., Syn, N. L., Wee, C. F., Tan, B. K. J., Wong, R. C., Chai, P., Kojodjojo, P., Kong, W. K., Lee, C.-H., Sia, C.-H., & Yeo, T.-C. (2022). Prevalence, types and treatment of bradycardia in obstructive sleep apnea – A systematic review and meta-analysis. Sleep Medicine, 89, 104-113. https://doi.org/10.1016/j.sleep.2021.12.003
- Tietjens, J. R., Claman, D., Kezirian, E. J., De Marco, T., Mirzayan, A., Sadroonri, B., Goldberg, A. N., Long, C., Gerstenfeld, E. P., & Yeghiazarians, Y. (2019). Obstructive sleep apnea in cardiovascular disease: A review of the literature and proposed multidisciplinary clinical management strategy. Journal of the American Heart Association, 8(1). https://doi.org/10.1161/jaha.118.010440
- Wolf, J., Drozdowski, J., Czechowicz, K., Winklewski, P. J., Jassem, E., Kara, T., Somers, V. K., & Narkiewicz, K. (2016). Effect of beta-blocker therapy on heart rate response in patients with hypertension and newly diagnosed untreated obstructive sleep apnea syndrome. International Journal of Cardiology, 202, 67-72. https://doi.org/10.1016/j.ijcard.2015.08.139
- Wolf, S., Wolf, C., Cattermole, T. C., Rando, H. J., Denino, W. F., Iribarne, A., Ross, C. S., Ramkumar, N., Gelb, D. J., Bourcier, B., Westbrook, B. M., & Leavitt, B. J. (2022). Cardiac surgery outcomes: A case for increased screening and treatment of obstructive sleep apnea. The Annals of Thoracic Surgery, 113(4), 1159-1164. https://doi.org/10.1016/j.athoracsur.2021.04.046
- Yeghiazarians, Y., Jneid, H., Tietjens, J. R., Redline, S., Brown, D. L., El-Sherif, N., Mehra, R., Bozkurt, B., Ndumele, C. E., & Somers, V. K. (2021). Obstructive sleep apnea and cardiovascular disease: A scientific statement from the american heart association. Circulation, 144(3). https://doi.org/10.1161/cir.0000000000000988
- Zhai, T., Liu, B., Zhang, J., & Wu, Y. (2022). Impact of obstructive sleep apnea on aortic disease occurrence: A meta-analysis. Heliyon, 8(8), e10049. https://doi.org/10.1016/j.heliyon.2022.e10049
- Zhang, L., Fu, M., Xu, F., Hou, F., & Ma, Y. (2019). Heart rate dynamics in patients with obstructive sleep apnea: heart rate variability and entropy. Entropy, 21(10), 927. https://doi.org/10.3390/e21100927
- Zwillich, C., Devlin, T., White, D., Douglas, N., Weil, J., & Martin, R. (1982). Bradycardia during sleep apnea. characteristics and mechanism. Journal of Clinical Investigation, 69(6), 1286-1292. https://doi.org/10.1172/jci110568
About the Author
I am a sophomore studying neuroscience with scientific interests revolving around heart health, neurodegenerative disease, and sleep. Sudden Cardiac Arrest is one of the leading causes of death in the United States, and is most likely to occur between the hours of 10 PM and 6 AM. I am fascinated by the emerging evidence that our sleep is deeply connected to our heart health, and look forward to researching specific associations between arrhythmias and sleep disorders as I progress throughout my higher education.
Cite this Article
Nguyen, S. and Patel, J. (2024). Retrospective Analysis of the relationship between Obstructive Sleep Apnea and Nocturnal bradycardia. University of Rochester, Journal of Undergraduate Research, 23(1). https://doi.org/10.47761/ZTSU1276
JUR | Creative Commons Attribution 4.0 BY International License
