Molecular Genetics
Investigating the Relationship of LINE-1 Expression and SIRT6 Rescue Across Rodent Species
Fall 2024, Volume 23, Issue 1
Natasha Sieczkiewicz ’26, John Martinez, Vera Gorbunova, Andrei Seluanov*
https://doi.org/10.47761/QSTU1242
Introduction
This experiment builds off of previous research investigating DNA double-strand break repair in rodent species by the protein SIRT6 (Tian et al., 2019). This study determined that in longer lived rodent species, SIRT6 has been optimized to repair double strand breaks, indicating its potential importance in the study of longevity. SIRT6 is a type of protein known as a sirtuin, which plays a role in regulating cellular processes such as aging, DNA repair, metabolism, and inflammation (Guo et al., 2022). SIRT6 acts as a deacetylase, mono-ADP-ribosyltransferase, and long fatty deacylase, engaging in diverse cellular signaling pathways ranging from the initial stages of DNA damage repair to disease advancement (Guo et al., 2022). These properties of SIRT6 cause DNA wrapped around histones to tighten, leading to more heterochromatin regions of the genome. SIRT6 also has anti-inflammatory properties, as it can suppress cytokines, which are small signaling molecules that help to regulate immune responses (Liu et al., 2020). SIRT6 helps to mitigate inflammation and maintain immune homeostasis, thereby contributing to healthy aging (Zhang et al., 2020). To understand the effects of SIRT6 on aging in rodent species, the aging of knockout SIRT6 mice can be monitored.
SIRT6 expression is directly linked to the expression of transposable element LINE-1 (L1) (Simon et al., 2019). In mammalian genes, the presence of this element is extensive, comprising approximately 20% of the genomic DNA in mice and humans (Lander et al., 2001). Because of the ability of transposable elements to cut and paste within a host’s genome, L1 activity causes breakages in DNA, which results in serious consequences for the host. While previous research on L1s has focused on their activity in the germ line, recent evidence has suggested that L1 activity in somatic tissues contributes to a number of age-related diseases, such as neurodegeneration and cancer (Simon et al., 2019). Overexpression of L1 is linked to inflammation, so eukaryotic cells have adapted ways to prevent it. Cells commonly use the SIRT6 protein to regulate expression of L1: it functions by employing the KAP1 protein, which contributes to the packaging of LINE1 in heterochromatin and thus preventing expression (Van Meter et al., 2014). Previous studies have found that mice deficient in the SIRT6 protein have higher levels of L1 expression, leading to shortened lifespan and chronic inflammation (Simon et al., 2019).
SIRT6 and L1 expression studies also highlight the differences in expression between longer lived and shorter lived rodent species. Rodents have drastically different life spans that are not specifically correlated to size, for example, Rats can live up to 4 years, whereas Naked Mole Rats can live for up to 30 (AnAge: The Animal Ageing and Longevity Database, n.d.). The considerable difference between these two maximum lifespans makes rodent species good candidates for cross-species experiments. The similarity in rodent genomes also allows for successful plasmid incorporation from different species. This experiment tested the success of the rescue of SIRT6 plasmid by using multiple species of rodents’ SIRT6. These plasmids were created using the same endogenous CMV promoter, because it induces very strong expression SIRT6 rescue functions by incorporating a SIRT6 plasmid into knockout mouse embryonic fibroblasts (MEFs) with the use of lipofectamine reagent. The goal of this experiment was to measure the change in SIRT6 production in these MEFs after transfection by different rodent species plasmids with varying lifespans. By transfecting these knockout MEFs with a plasmid vector, they can successfully uptake the new species SIRT6 plasmid into the host cell genome, where it can be expressed and therefore “rescue” SIRT6 protein production.
By using multiple species for this experiment, conserved biological mechanisms of the L1 repression can be better understood for its effect on lifespan. Using a cross-species study also highlights the differences in an animal’s ability to rescue the SIRT6 protein when introduced as a plasmid. Differences in maximum lifespan are supported by a species’ ability to rescue SIRT6, linking aging to the expression of this protein. Comparing data of SIRT6 knockout DNA from multiple species supports prior research on the direct relation between a species’ ability to produce or rescue SIRT6 and their maximum lifespan. Furthermore, investigation of the relationship between SIRT6 and maximum lifespan of multiple species can allow the understanding of aging to be better translated to humans.
Methods
Cell Passaging
This investigation utilized cell culture techniques to cultivate and maintain cells of multiple species. The mouse embryonic fibroblasts, MEFs, were plated on two separate 10 cm plates, one wild type (WT) and one SIRT6 knockout, using Dulbecco’s Modified Eagle Medium (DMEM) These MEF lines were used through the length of this experiment. First, the DMEM was aspirated out, and the cells were washed with PBS. Then, cells were trypsinized, and placed in an incubator for 3-5 minutes. When the majority of cells were no longer confluent, the trypsin was inactivated with (DMEM) and the cells were collected and spun down for 5 minutes to create a pellet. The supernatant was aspirated off and the pellet was resuspended in 1 mL of DMEM. The pellet was then broken and a cell count was recorded.
Transfections
Over the course of this experiment, SIRT6 MEFs were passaged and plated onto 6-well plates and multiple IF slides. They were then transfected with the following species’ SIRT6 plasmid, one per well: Hampster, Deer Mouse, Mouse Porcupine, Beaver, Naked Mole Rat, Red Squirrel, Paca, Capybara, Gerbil, Guinea Pig, Rat, and Human. The Mouse MEFs were transfected with Lipofectamine and Opti-Mem. First, Tube A was prepared using a 3:100 ratio of Lipofectamine and Opti-Mem. A Tube B was prepared for each of the plasmids with the following: 250 uL of Opti-Mem, 5 uG plasmid DNA, and 5µL of P3000 reagent. Tube A was distributed evenly to each Tube B and left to incubate for 10 minutes at room temperature, after which 250uL of each tube B was added to each well.
RNA Isolation
The cells collected in transfection then underwent further RNA isolation and purification techniques. MEF cells were collected from plates using cell passaging methods, and centrifuged to form a pellet. The supernatant was poured off, and the pellet was left to air dry. The RNA of each plasmid was isolated and purified with the Addgene kit.This process included washing the pellet with multiple buffers and spinning them down to first isolate RNA. After this series of washes, the RNA was eluted using a spin column, it was collected and the concentration determined, and the samples were stored at -20℃.
cDNA synthesis for RT-qPCR
The isolated RNAwas then used for Superscript III cDNA synthesis. For each species, 5µg of the isolated RNA was mixed with 1µL of dNTPS and 1µL of OligodT primer in a labeled PCR tube. This was briefly spun down and incubated at room temperature for 5 minutes. Separately, 2µL of each DTT and 10x Buffer were mixed with 4µL MgCl2. Then, 1µL of the enzymes RNaseOUT and Superscript III were added. The second mixture was added to each of the tubes with RNA and mixed. The tubes were incubated in the PCR block at 50℃ for 50 minutes, then 85℃ for five minutes, and finally 12℃ for five minutes. An aliquot of RNaseH was diluted with ddH2O in a 1:1 ratio; 2µl of this was added to each sample, and PCR tubes were incubated for 30 minutes at 37℃. The samples were diluted as needed, using ddH2O to achieve qPCR dilutions. The samples were stored at -20℃ until they were analyzed using RT-qPCR.
Immunofluorescence Staining
The IF slides for each species were then fixed and stained. Samples were aspirated and then washed twice with cold PBS. The slides were fixed with 3.7% PFA (4℃) and placed in an incubator (37℃, 5% CO2) for 10 minutes. The slides were then aspirated and washed 3 times with PBS. Then, 0.5% Triton X-100 was added and each slide was left on the shaker at room temperature for 10 minutes. Slides were washed 4 times with PBS for 10 minutes on a shaker. 2 mL of 100% ice-cold methanol was added to each slide and placed in a -20℃ freezer overnight. The slides were washed with PBS, and a blocker (10% FBS and 5% BSA in PBS) was added. Subsequently, the slides were placed on the shaker for 1 hour. The primary antibody was added and left to shake at 4℃ overnight. Next, the cells were washed with 0.1% Triton X-100 4 times at room temperature on a shaker for 10 min each.
The secondary antibody (1:1000 in the blocker) was added, and left to shake for one hour at room temperature,covered. The cells were washed with PBS for 10 minutes on a shaker at room temperature 4 times. The PBS was first removed, followed by the chambers from the slides. After drying, 30 µl of DAPI mounting medium was added to each chamber and a cover slip was placed on top for storage at -80℃.
Results and Discussion
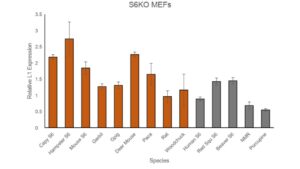
This experiment further validated data gathered from previous experiments; SIRT6 expression decreases LINE1 expression in mouse MEFs. These experiments also further validated that SIRT6, the longevity regulating protein, is a powerful repressor of L1 activity (Van Meter et al., 2014). The qPCR results, though not significant, suggest that longer lived species, on average, have lower relative L1 expression (Figure 1.1).
Figure 1.1: Results of qPCR on multiple species S6 plasmid incorporated into SIRT6 knockout MEF’s. Longer lived species are gray, and shorter lived species are rust color. NMR refers to the naked mole rat. The graph shows relative L1 expression once successful transfection of new species SIRT6 plasmid. Standard error bars included. Highest expression is in Hamster at around 2.75. Lowest expression is in Porcupine at around 0.6.
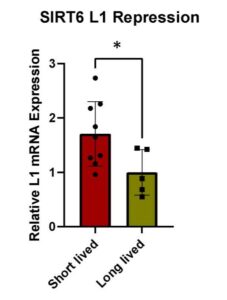
While there are some outliers, such as the Rat, a short lived species, the general pattern of Figure 1.1 supports the conclusion that the longer a species’ maximum lifespan is, the lower the L1 expression. This experiment validated this throughout multiple rodent species, as well as confirmed the relationship between protein SIRT6 and L1 expression. SIRT6 attaches to the 5′-UTR of L1 loci, where it performs mono-ADP ribosylation on the nuclear corepressor protein, KAP1 (Van Meter et al., 2014). This modification aids in KAP1’s interaction with the heterochromatin factor, thereby assisting in the formation of repressed heterochromatin around L1 elements (Van Meter et al., 2014). Decreased L1 expression is due to the increase of repressed heterochromatin in the regions surrounding these L1 elements, which occurs when SIRT6 is expressed at a higher level. Short lived species have significantly higher L1 expression (p = 0.0368) than longer lived species (Figure 1.2), which is likely due to the ability of MEF’s to rescue SIRT6 from other species. This further supports the conclusion that longer lived species are more effective at repressing L1 transposons through production of SIRT6
Figure 1.2: Condensed results from qPCR run. Each black dot represents a different species’ S6 plasmid, and trends grouped together in larger boxes. Relative L1 mRNA expression vs longevity of species measured. Standard error bars included. Lower levels of expression indicate higher levels of SIRT6 L1 repression. P value is 0.0368, indicating significance and rejecting null hypothesis.
Figure 1.3: Images, common names, latin names, and maximum lifespans of plasmids of each species used. Four longer lived species: Beaver, Porcupine, Red Squirrel, and Naked Mole Rat. 8 shorter lived species above: Mouse, Hamster, Gerbil, Rat, Guinea Pig, Paca, Capybara, Woodchuck. Images shown for relative size and appearance of each species. Longest maximum lifespan is the Naked Mole Rat, and shortest maximum lifespan is in the Hamster (AnAge: The Animal Ageing and Longevity Database, n.d.)..
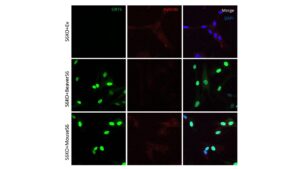
This is further supported by the immunofluorescence staining of rescue SIRT6 MEFs, from prior experimentation (Figure 2.1) and this experiment (Figure 2.2). In both figures, the shorter lived rodent species had higher hybrid presence, indicating L1 expression in these MEFs (John Martinzed, unpublished). Additionally, both shorter lived species SIRT6, the Hamster and Mouse, were not rescued as successfully by the MEFs. These stains were compared to a negative control, where no SIRT6 plasmid was incorporated. Both the Hamster and Mouse show similarities to the negative control; there is a significantly higher presence of hybrid proteins compared to longer lived species (Figure 2.1), indicating that SIRT6 was not successfully rescued, and L1 expression occurred at relatively the same rate as the negative control. In both longer lived species, the rescue of SIRT6 was higher (Figure 2.1 & 2.2), and the hybrid protein levels were lower. Additionally, both Beaver and Porcupine SIRT6 were more present in the images, suggesting a successful rescue. These results indicate that something about short lived species’ SIRT6 proteins are less effective at L1 repression than longer lived species’ SIRT6 proteins.
Figure 2.1: Previous experimental Immunofluorescence of mouse and beaver SIRT6 in S6 knockout mice. Beaver SIRT6 is more hyperphosphorylated than mouse and is better at repressing L1s IF for SIRT6, DNA/RNA hybrids, and DAPI in human fibroblasts knocked out for SIRT6. Top row is stably-transfected with either empty vector, or mouse SIRT6, shown by prevalence of staining. Mouse knockout shows less present SIRT6 (green) and prevalence of hybrid. (John Martinzed, unpublished)
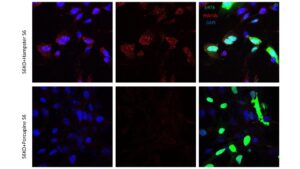 Figure 2.2: New experimental data with a new long and short lived species. Immunofluorescence of Porcupine S6 vs Hamster S6. Staining shows protein DAPI (blue) and SIRT6 transfection (green), and hybrid RNA present after S6 transfection. S6KO Hamster MEFs depict higher presence of hybrids compared to the S6KO Porcupine. Porcupine also shows higher prevalence of SIRT6 protein (green) than the Hamster. Immunofluorescence of other species was not obtained nor published.
Figure 2.2: New experimental data with a new long and short lived species. Immunofluorescence of Porcupine S6 vs Hamster S6. Staining shows protein DAPI (blue) and SIRT6 transfection (green), and hybrid RNA present after S6 transfection. S6KO Hamster MEFs depict higher presence of hybrids compared to the S6KO Porcupine. Porcupine also shows higher prevalence of SIRT6 protein (green) than the Hamster. Immunofluorescence of other species was not obtained nor published.
It is important to understand the differences in the ability of SIRT6 to repress L1 expression across species and how this could be correlated to lifespan. DNA damage is a cause of aging, and organisms that are able to better repair this damage are likely to live longer The differences can be attributed to variations in the structure, expression levels, and enzymatic activity of SIRT6 across different species. Additionally, genetic variations or mutations in the SIRT6 gene among different species may result in alterations in its ability to bind to L1 loci or modify associated proteins, ultimately influencing the efficacy of L1 repression (Simon et al., 2019). Furthermore, differences in the regulatory mechanisms or interacting partners of SIRT6 in different species could also contribute to variations in L1 repression (Simon et al., 2019). Based on previous research and supported by this experiment, it can be confirmed that longer-lived species have more effective and abundant SIRT6 proteins present in their cells. This is likely an evolutionary adaptation; mammals with longer maximum life spans have better adapted SIRT6 expression to decrease L1 expression to reduce chronic inflammation and DNA damage, and therefore are able to live longer. Future work will consist of repeating transfection experiments with more non-rodent species SIRT6 plasmids. Additionally, more IF slide samples of other short and long lived rodent species should be stained and imaged to confirm these conclusions.
References
AnAge: The Animal Ageing and Longevity Database. (n.d.). Genomics.senescence.info. https://genomics.senescence.info/species/index.html
Guo, Z., Li, P., Ge, J., & Li, H. (2022). SIRT6 in Aging, Metabolism, Inflammation and Cardiovascular Diseases. Aging and Disease, 13(6), 1787. https://doi.org/10.14336/ad.2022.0413
Liu, G., Chen, H., Liu, H., Zhang, W., & Zhou, J. (2020). Emerging roles of SIRT6 in human diseases and its modulators. Medicinal Research Reviews, 41(2), 1089–1137. https://doi.org/10.1002/med.21753
Simon, M., Van Meter, M., Ablaeva, J., Ke, Z., Gonzalez, R. S., Taguchi, T., De Cecco, M., Leonova, K. I., Kogan, V., Helfand, S. L., Neretti, N., Roichman, A., Cohen, H. Y., Meer, M. V., Gladyshev, V. N., Antoch, M. P., Gudkov, A. V., Sedivy, J. M., Seluanov, A., & Gorbunova, V. (2019). LINE1 Derepression in Aged Wild-Type and SIRT6-Deficient Mice Drives Inflammation. Cell Metabolism, 29(4), 871-885.e5. https://doi.org/10.1016/j.cmet.2019.02.014
Tian, X., Firsanov, D., Zhang, Z., Cheng, Y., Luo, L., Tombline, G., Tan, R., Simon, M., Henderson, S., Steffan, J., Goldfarb, A., Tam, J., Zheng, K., Cornwell, A., Johnson, A., Yang, J.-N., Mao, Z., Manta, B., Dang, W., & Zhang, Z. (2019). SIRT6 Is Responsible for More Efficient DNA Double-Strand Break Repair in Long-Lived Species. Cell, 177(3), 622-638.e22. https://doi.org/10.1016/j.cell.2019.03.043
Van Meter, M., Kashyap, M., Rezazadeh, S., Geneva, A. J., Morello, T. D., Seluanov, A., & Gorbunova, V. (2014). SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nature Communications, 5, 5011. https://doi.org/10.1038/ncomms6011
Zhang, X., Zhang, R., & Yu, J. (2020). New Understanding of the Relevant Role of LINE-1 Retrotransposition in Human Disease and Immune Modulation. Frontiers in Cell and Developmental Biology, 8. https://doi.org/10.3389/fcell.2020.00657
About the Author
Natasha Sieczkiewicz (she/her) is a junior at the University of Rochester from Lewisburg, Pennsylvania. She is majoring in Molecular Genetics with a minor in Anthropology, and a cluster in bioethics which she hopes to use to expand her knowledge on the effects of different genetic disorders on various populations. Outside of classes, she can be found conducting aging research in the Gorbunova lab, or TAing introductory biology courses such as genetics and Bio 111. In her free time she enjoys going on walks, reading, and spending time with her cats.
Cite this Article
Sieczkiewicz, N., Martinez, J., Gorbunova, V., Seluanov, A. (2024). Investigating the Relationship of LINE-1 Expression and SIRT6 Rescue Across Rodent Species. University of Rochester, Journal of Undergraduate Research, 23(1). https://doi.org/10.47761/QSTU1242
JUR | Creative Commons Attribution 4.0 BY International License