New research directs charges through single molecules
Scientists are facing a number of barriers as they try to develop circuits that are microscopic in size, including how to reliably control the current that flows through a circuit that is the width of a single molecule.
Alexander Shestopalov, an assistant professor of chemical engineering at the University of Rochester, has done just that, thereby taking us one step closer to nanoscale circuitry.
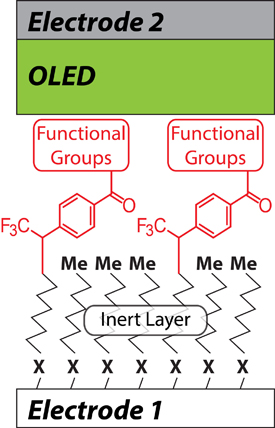
“Until now, scientists have been unable to reliably direct a charge from one molecule to another,” said Shestopalov. “But that’s exactly what we need to do when working with electronic circuits that are one or two molecules thin.”
Shestopalov worked with an OLED (organic light-emitting diode) powered by a microscopically small, simple circuit in which he connected a one-molecule thin sheet of organic material between positive and negative electrodes. Recent research publications have shown that it is difficult to control the current traveling through the circuit from one electrode to the other in such a thin circuit. As Shestopalov explains in a paper published in the journal Advanced Material Interfaces, the key was adding a second, inert layer of molecules.
The inert—or non-reactive—layer is made of a straight chain of organic molecules. On top a layer of aromatic—or ring-shaped—molecules acts like a wire conducting the electronic charge. The inert layer, in effect, acts like the plastic casing on electric wires by insulating and separating the live wires from the surrounding environment. Since the bottom layer is not capable of reacting with the overlapping layer, the electronic properties of the component are determined solely within the top layer.
The bi-layer arrangement also gave Shestopalov the ability to fine-tune his control of the charge transfer. By changing the functional groups—units of atoms that replace hydrogen in molecules and determine a molecule’s characteristic chemical reactivity—he could more precisely affect the rate at which the current moved between the electrodes and the upper layer of organic molecules.
In molecular electronic devices, some functional groups accelerate the charge transfer, while others slow it down. By incorporating the inert layer of molecules, Shestopalov was able to reduce any interference with the top layer and, as a result, achieve the precise charge transfer needed in a device by changing the functional group.
For example, an OLED may need a faster charge transfer to maintain a specific luminescence, while a biomedical injection device may require a slower rate for delicate or variable procedures.
While Shestopalov overcame a significant obstacle, there remains a great deal of work to be done before bi-layer molecular electronic devices become practical. The next obstacle is durability.
“The system we developed degrades quickly at high temperatures,” said Shestopalov. “What we need are devices that last for years, and that will take time to accomplish.”
Shestopalov’s research was funded by the National Science Foundation and University of Rochester ChemE Startup.