Most laypersons are familiar with the three states of matter as solids, liquids, and gases. But there are other forms that exist. Plasmas, for example, are the most abundant form of matter in the universe, found throughout our solar system in the sun and other planetary bodies. Scientists are still working to understand the fundamentals of this state of matter, which is proving to be ever more significant, not only in explaining how the universe works but in harnessing material for alternative forms of energy.
For the first time, researchers at the University of Rochester’s Laboratory for Laser Energetics (LLE) have found a way to turn a liquid metal into a plasma and to observe the temperature where a liquid under high-density conditions crosses over to a plasma state. Their observations, published in Physical Review Letters, have implications for better understanding stars and planets and could aid in the realization of controlled nuclear fusion—a promising alternative energy source whose realization has eluded scientists for decades. The research is supported by the US Department of Energy and the National Nuclear Security Administration.
What is a plasma?
Plasmas consist of a hot soup of free moving electrons and ions—atoms that have lost their electrons—that easily conducts electricity. Although plasmas are not common naturally on Earth, they comprise most of the matter in the observable universe, such as the surface of the sun. Scientists are able to generate artificial plasmas here on Earth, typically by heating a gas to thousands of degrees Fahrenheit, which strips the atoms of their electrons. On a smaller scale, this is the same process that allows plasma TVs and neon signs to “glow”: electricity excites the atoms of a neon gas, causing neon to enter a plasma state and emit photons of light.
From a liquid to a plasma
As Mohamed Zaghoo, a research associate at the LLE, and his colleagues observed, however, there is another way to create a plasma: under high density conditions, heating a liquid metal to very high temperatures will also produce a dense plasma. “The transition to the latter has not been observed scientifically before and is precisely what we did,” Zaghoo says.
One of the unique aspects of this observation is that liquid metals at high densities exhibit quantum properties; however, if they are allowed to cross over to the plasma state at high densities, they will exhibit classical properties. In the 1920s, Enrico Fermi and Paul Dirac, two of the founders of quantum mechanics, introduced the statistical formulation that describes the behavior of matter made out of electrons, neutrons, and protons—normal matter that makes up the objects of Earth. Fermi and Dirac hypothesized that at certain conditions—extremely high densities or extremely low temperatures—electrons or protons have to assume certain quantum properties that are not described by classical physics. A plasma, however, does not follow this paradigm.
In order to observe a liquid metal crossing over to a plasma, the LLE researchers started off with the liquid metal deuterium, which displayed the classical properties of a liquid. To increase the density of the deuterium, they cooled it to 21 degrees Kelvin (-422 degrees Fahrenheit). The researchers then used the LLE’s OMEGA lasers to set off a strong shockwave through the ultracool liquid deuterium. The shockwave compressed the deuterium to pressures up to five million times greater than atmospheric pressure, while also increasing its temperatures to almost 180,000 degrees Fahrenheit. The sample started off completely transparent, but as the pressure rose, it transformed into a shiny metal with high optical reflectivity.
“By monitoring the reflectance of the sample as a function of its temperature, we were able to observe the precise conditions where this simple lustrous liquid metal transformed into a dense plasma,” Zaghoo says.
Understanding matter at extreme conditions
The researchers observed that the liquid metal initially exhibited the quantum properties of electrons that would be expected at extreme temperatures and densities. However, “at about 90,000 degrees Fahrenheit, the reflectance of the metallic deuterium started rising with a slope that is expected if the electrons in the system are no longer quantum but classical,” Zaghoo says. “This means that the metal had become a plasma.”
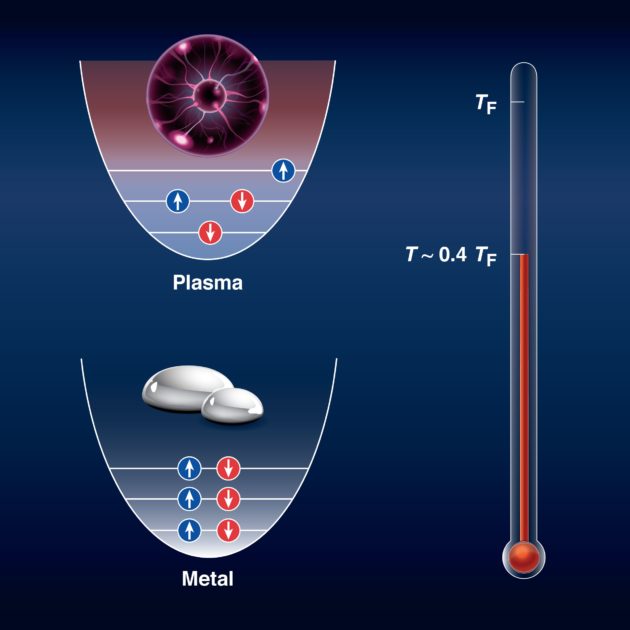
That is, the LLE researchers started off with a simple liquid. Increasing the density to extreme conditions made the liquid enter a state where it exhibited quantum properties. Raising the temperature even further made it turn into a plasma, at which point it exhibited classical properties, yet was still under high-density conditions, says Suxing Hu, a senior scientist at LLE and a co-author on the study. “What is remarkable is that the conditions at which this crossover between quantum and classical occurs is different from what most people expected based on plasma textbooks. Furthermore, this behavior could be universal to all other metals.”
Understanding these fundamentals of liquids and plasmas allows researchers to develop new models to describe how materials at high densities conduct electricity and heat, and can help explain matter in the extremes of the solar system, as well as help in attaining fusion energy, Zaghoo says. “This work is not just a laboratory curiosity. Plasmas comprise the vast interiors of astrophysical bodies like brown dwarfs and also represent the states of matter needed to achieve thermonuclear fusion. These models are essential in our understanding of how to better design experiments to achieve fusion.”
Read more

With data science, Rochester’s laser lab moves closer to controlled nuclear fusion
One of the biggest challenges to controlled nuclear fusion has been the lack of accurate models to predict increased fusion energy yields. Now a Rochester team of more than 50 scientists has used “big data” to triple fusion yields.
What is fusion, and why is it so difficult to create?
“All the stars, including the sun, are powered by fusion. We are here because of fusion. But fusion is really hard to create,” says E. Michael Campbell, director of the Laboratory for Laser Energetics.