A multidisciplinary collaboration will create a new light-sheet microscope on campus, allowing 3D imaging of complex cellular structures.
A new multidisciplinary collaboration between the University of Rochester’s departments of biology, biomedical engineering, and optics and the Goergen Institute for Data Science will establish an innovative microscopy resource on campus, allowing for cutting-edge scientific research in biological imaging.
Michael Welte, professor and chair of the Department of Biology, is the lead principal investigator of the project, which was awarded a $1.2 million grant from the Arnold and Mabel Beckman Foundation.
“The grant supports an endeavor at the intersection of optics, data science, and biomedical research, and the University of Rochester is very strong in these areas,” Welte says. “The University has a highly collaborative culture, and the close proximity of our college and medical center makes Rochester ideally suited to lead advances in biological imaging.”
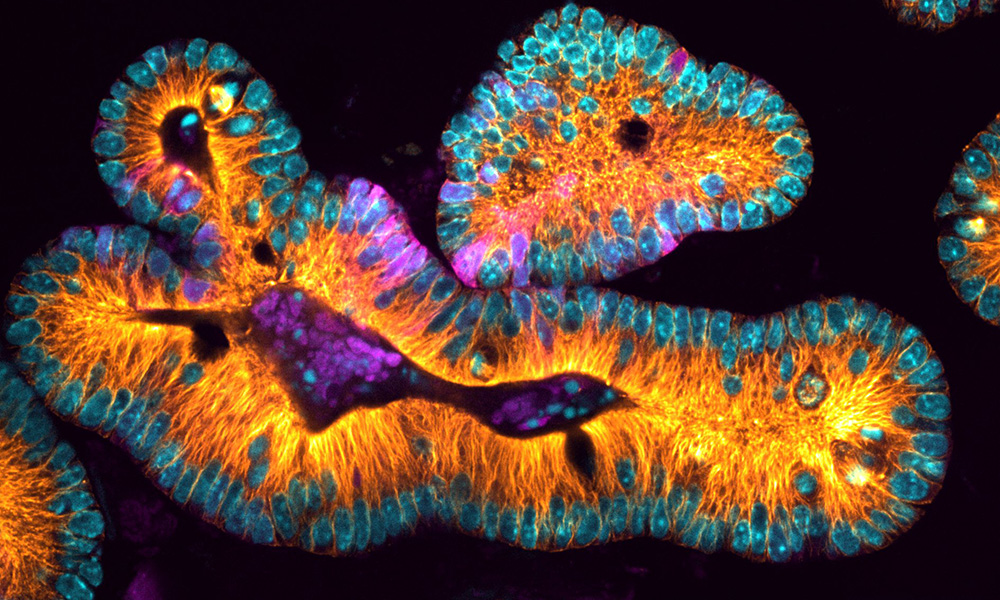
The project will include developing and building a novel light-sheet microscope that employs freeform optical designs devised at Rochester. The microscope, which will be housed in a shared imaging facility in Goergen Hall and is expected to be online in 2022, enables three-dimensional imaging of complex cellular structures in living samples. Researchers and engineers will continually improve the microscope, and it will eventually become a resource for the entire campus research community.
“The optical engineers working on this project will take light-sheet technology into new domains,” says Scott Carney, professor of optics and director of Rochester’s Institute of Optics, who is a co-principal investigator on the project. “They will transform a precise, high-end microscope into a workhorse for biologists working at the cutting edge of their disciplines to make discoveries about the very fabric of life at the cellular and subcellular level.”
The microscope will produce large amounts of data that will require new methods to better collect, analyze, and store the images.
“These efforts will focus on developing algorithms for computational optical imaging and automated biological image analysis, as well as on big data management,” says Mujdat Cetin, a professor of electrical and computer engineering and the Robin and Tim Wentworth Director of the Goergen Institute for Data Science. Cetin is also a co-principal investigator on the project.
While many other research microscopes illuminate objects pixel by pixel, light-sheet technology illuminates an entire plane at once. The result is faster imaging with less damage to samples, enabling researchers to study biological processes in ways previously out of reach.
In addition to funding the construction of the microscope and development of the data science component, the grant from the Arnold and Mabel Beckman Foundation supports three biological research projects:
- Anne Meyer, an associate professor of biology, will study how communities of bacteria interact and change inside lab-grown biofilms—three-dimensional structures that mimic how bacteria (including bacteria that cause disease) live in nature. Most microscopy is so slow that the bacteria may have already divided by the time the microscope is back at the same spot. The speed of light-sheet microscopy is expected to enable Meyer to see the bacteria in the 3D space in real time.
- Dan Bergstralh, an assistant professor of biology, will study how the behavior of single cells generates the 3D structure of animal organs. Using normal microscopy, researchers can either study single cells and their properties or the properties of tissues and organs. Light-sheet microscopy will allow Bergstralh to image both cells and organs at enough detail to understand how they influence each other.
- Jim McGrath, a professor of biomedical engineering, and Richard Waugh, professor of biomedical engineering and vice provost for research, will study how molecules and cells travel from the bloodstream into surrounding tissue. Traditional microscopy is insufficient to visualize critical aspects of this transport, but the new microscope will allow the researchers to look across a whole plane of many cells at the same time. It will also allow them to add drugs during imaging and immediately see how the sample reacts.
“Not only am I excited about each of the individual projects—from intimate looks at bacteria to finding new ways to analyze images—I am absolutely thrilled about the prospect of building something even bigger and better via the close collaboration of disciplines Rochester excels at individually: optics, data science, and biomedical research,” Welte says. “I believe this joint endeavor is only the first in a long line that will establish Rochester as a leader in biological imaging.”