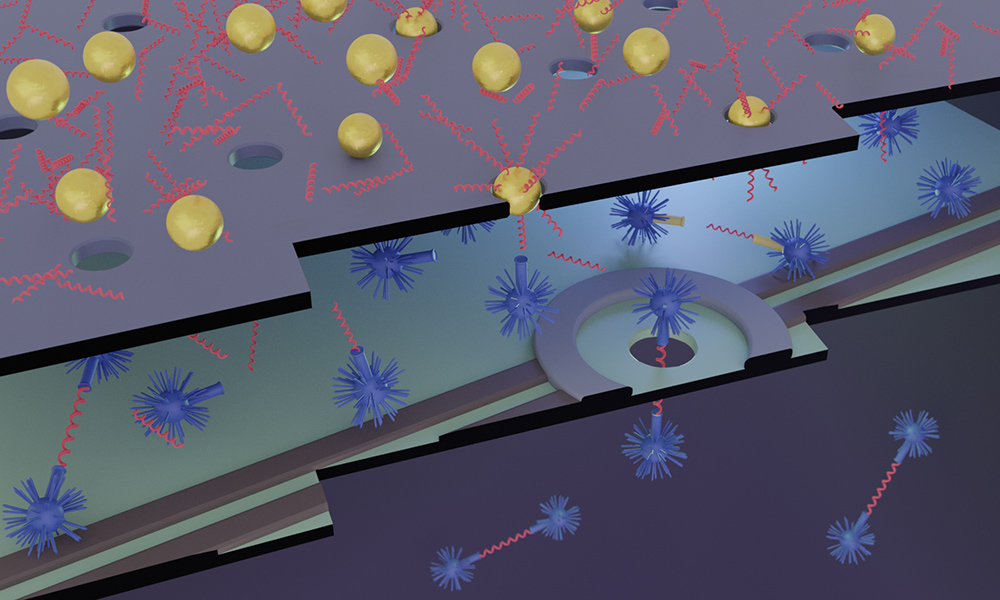
Researchers at Rochester and Ottawa are developing a compact, portable, and cost-effective detection device.
The tiny proteins that can alert us to some of the most acute human disorders—traumatic brain injury (TBI), cancer, heart attack, and stroke—are freely circulating in our blood, urine, and other bodily fluids. But detecting these tiny “biomarkers” is problematic and costly.
Biomarkers are not only tiny but very dilute. The most direct approach to detecting them involves sending serum samples off to undergo a sensitive but costly so-called dELISA (digital enzyme linked immunosorbent assay) test. For some medical conditions such as TBI, an alternative—also costly—is to rely on CT and MRI scans of the affected organs.
With the support of a $1.6 million grant from the National Institute of Biomedical Imaging and Bioengineering, researchers at the University of Rochester and the University of Ottawa are developing a far less expensive, faster, portable alternative—a biomarker detector using nanopore membranes and filters so thin it would take more than 1,000 stacked on top of each other to equal the width of a human hair.
The device would be just as accurate, but inexpensive enough for doctor’s offices and smaller hospitals to afford. And it would be portable and nimble enough to produce results in a matter of minutes when used in the field by emergency responders, says Jim McGrath, a professor of biomedical engineering at Rochester, who is co-leading the project with Vincent Tabard-Cossa, associate professor of physics at the University of Ottawa.
“The key is that we’re making a digital sensing platform that is fully electronic. We eliminate the lasers and optics of the existing commercial platforms and use a single nanopore to count the biomarkers as they pass through,” McGrath says.
Co-principal investigator Jonathan Flax, a research assistant professor of urology and biomedical engineering at the University of Rochester Medical Center, says that although the device could ultimately be used to detect a broad range of disorders, “we had an overriding goal at this point to show a proof of concept for two different levels of detection, in two types of biofluids.” The focus is on detecting biomarkers of TBI, which can be found in blood, and the biomarkers for predicting the response of bladder cancer to immunotherapy, which can be found in urine.
Diagnostic levels of biomarkers for both conditions have been clearly established in previous studies, including a seminal Lancet paper by co-investigator Jeff Bazarian, a professor of emergency medicine and neurology at Rochester and a leading TBI expert. The research will make it easier to compare the device’s ability to detect biomarkers directly against the results obtained by dELISA platforms—specifically, the researchers note, the Quanterix SiMoA.
Two types of nanopore membranes working in concert
By combining two very different types of membranes, the researchers are able to not only detect biomarkers, but convert them into DNA “proxies” that in turn are further concentrated, thereby greatly accelerating the process.
Otherwise, the detection of the most dilute biomarkers would take years,” McGrath says. “By concentrating 1,000, 10,000, even 100,000 times, we can bring that down to minutes.”
McGrath’s lab has helped pioneer the development of thin membranes with millions and even billions of nanopores for use as filters and sensors. They are building the membranes that concentrate the particles. Tabard-Cossa’s lab, including co-principal investigator Michel Godin, an associate professor of physics, is expert in creating membranes with a single nanopore opening that can detect each DNA proxy passing through it. Tabard-Cossa’s lab is developing the technology to count the proxies.
SiMPore, a University of Rochester spin-out company that manufactures ultrathin membranes in the Rochester area, will build a new chip that integrates the two types of membranes in one device. And Flax, who is also an expert in molecular biology, is assisting with designing the various assays (analyses used to determine the presence and amounts of a substance) being used at each step of development and testing.
McGrath says he is excited to be working with Flax and the Ottawa team. “This technology and team give us a very good shot at making a new type of assay that find its way into clinics.”
Read more
 Scientist’s accidental exhale leads to improved DNA detector
Scientist’s accidental exhale leads to improved DNA detectorHow water vapor became integral to the development and design of a novel device for detecting the DNA biomarkers affiliated with disease.
 New computing device would let microprocessors go ‘all out’
New computing device would let microprocessors go ‘all out’Researcher Mohammad Kazemi has proposed an entirely new concept for computer architecture to overcome the problems of heat transfer inherent in traditional microprocessors.
 ‘Organ on a chip’ is the wave of the future
‘Organ on a chip’ is the wave of the futureRochester researchers are building technology to predict the course of tendon injuries—a form of personalized medicine that will lead to more effective treatments.